Electrons are now presumed to be a self contained atomic particle, not composed
of anything any smaller, and have a mass of 1/1836 of that of the proton. If you
could get 1836 of those guys glued together, the group would balance a proton on
the other end of a teeny tiny teeter totter. But you couldn’t do that because an
electron is something called a fermion which means only one can occupy a given
space at a time due to a rule called the Pauli exclusion principle and since
they all have a negative charge, they each push the others away from themselves.
The other 1835 guys would have to float around somewhere else, all of them
staying out of the way of each other. At the moment however, we don’t know of
any element that has that many electrons floating around the nucleus anyway so
the 1/1836 value is really only of academic interest. The electron has a charge
of -1.602 x 10-19 coulomb. Notice that the charge of the electron is negative.
Meanwhile, the charge of the much more massive proton, is +1.602 x 10-19
coulomb. So, charge-wise, the electron and proton are equivalent but have
opposite signs. When you smoosh one of each together, you get an annihilation of
both particles, a net charge of zero, and the release of gamma rays and photons.
Congratulations, you’ve just learned your first bit about quantum
electrodynamics. Not too bad was it?
Electrons are very mobile, light, and energetic particles. Protons on the other
hand are pretty heavy, comparatively, less energetic, and tend to stay put.
Since the number of protons in the nucleus of an atom of an element determine
the type of element, you don’t want protons moving around and being exchanged
between adjacent atoms. Otherwise, you would have one element changing into
another element unpredictably. You don’t want a dish of carbon changing into
part sulfur and part nitrogen at the drop of a hat. You want that elemental
carbon to stay as it is until purposely caused to change. On the other hand,
since the highly mobile electrons are easily attracted to other protons, those
electrons can be pretty easily shared with the protons in the nucleus of nearby
other elements. This behavior permits elements to combine into molecules of
compound chemicals by sharing electrons yet keeping the protons in the
compound’s elements intact.
The chemistry of elements and compounds comes into play in electronics all the
time. By changing the characteristics of chemicals involved in materials, we can
cause LEDs to emit different colors of light, cause transistors to have
different gain and loss characteristics, and cause different types of
transistors in integrated circuits to operate at different voltages and speeds
of transmission. Chemistry plays vital roles in all areas of electronics,
setting the characteristics of conducting and insulating materials as well.
Back to seeing what those electrons are capable of. Always remember that
electrons are negatively charged. When they move, they move from an area of a
concentrated negative charge to an area of positive (or less negative) charge.
This is really important to understand and remember. Life’s experiences sort of
direct you in the wrong way here, making you think that electricity flows from
the “+” (plus, or red) terminal to the “-” (minus or black) terminal. But no!
That’s not what happens. Its the other way around! When those mobile electrons
move, it causes a current to flow. Just like water flowing in a stream, the
electrons move from a point of higher negative charge to a point of lower
negative charge (or more positive) causing a stream of electron current.
Current flow is the result. From the minus terminal (usually labeled as black-
but not always) to the plus terminal (usually labeled as red, but again, not
always; you always have to be careful about this color labeling business).
Remember that the electric charge of a single electron is -1.602 x 10-19
coulomb. If we have 1×1019 electrons flowing from point A to point B, we will
have a charge difference of -1.602 coulomb between A and B. Similarly, if we
happened to have 6.2422 x 1018 electrons laying around and we got them all to
zoom past an arbitrary point in one second, we’d have exactly 1 ampere of
current flowing past that point. (6.2422 x 1018 x |-1.60210-19| = 1.0). If,
however, we had only 3.1211 x 1018 electrons, and it still took them that one
second to pass that arbitrary point, we’d only have half an ampere of current
flow. The charge of each electron remains constant but we have only half as many
electrons as before so the quantity of current flow is half as much. Current
flow then, measured and expressed in amperes, is defined as 1 coulomb of charge
flowing past a point in 1 second. To have current, you have to have movement of
the electrons and their associated charge.
That’s pretty simple. So, what’s a coulomb? The short answer is that it is the
arbitrary name given to a measure of the combined amount of electric charge on
6.2422 x 1018 electrons. The long answer is a bit more involved and puts us down
a rabbit hole that leads to a whole different world of stuff called atomic and
quantum theory. This is actually pretty cool stuff and all links together pretty
nicely but leads us into looking at some pretty scary mathematics.
6.2422 x 1018 electrons sounds like quite a few electrons. Is there some way to
get a feeling for how much that really is? Sure, let’s use an amount of iron to
see how big of a pile of it we would have to have to accumulate that many
electrons. Since iron has an atomic number or weight of 26, that means that a
single atom of iron has 26 protons in its nucleus. Since there are 26 protons,
there are also 26 electrons. If we divide 6.2422 x 1018 by 26 we have 2.4008 x
1017, still a pretty big number. Since electrons don’t have any mass, it is the
mass of the protons that is going to account for the mass in this accumulation
of iron. The mass of a proton is about 938 MeV/c2. Oops, that looks like it
isn’t going to be as straight forward as it was looking, I was hoping for grams.
But no. MeV by the way is million electron volts and c2 is the speed of light
squared.
938 million electron volts divided by the speed of light squared. Volts and the
speed of light? Well, we’ll get to volts in a little while so maybe we stumbled
across something useful. But the speed of light? What’s that got to do with it?
Well, you’ve probably heard of Albert Einstein and his famous equation E = mc2.
That equation established that mass and energy are directly proportional to each
other and related by the speed of light squared. What that means is, a given
mass of matter has a lot of energy in it, that mass and energy are equivalent,
and that mass and energy can be converted to each other. Those electron volts
are really a measure of the amount of energy contained within the proton
expressed in joules.
As we’ve just seen, its hard to talk about things in physics, electronics, and
chemistry without encountering that equal sign in Einstein’s equation. Mass and
energy are equivalent, we can’t have one without the other. The equivalence is
so common that it has been simplified to the value of 1 GeV/c2 = 1.783 x 10-27
kg. A really small mass of 1.783 x 10-27 kilograms is equivalent to 1 giga
electron volts accelerated to the speed of light squared.
Our proton mass then equates to 0.938 x 1.783 x 10-27 kg, 938 MeV/c2 divided by
1 GeV/c2 or 1.6725 x 10-27 kg per proton. Multiply that by 2.4008 x 1017
protons and our pile of iron should have a mass of 4.0153 x 10-10 grams. A
really small amount! A coulomb of electrons is contained within that tiny amount
of iron.
Lets stop for a minute and explain some things.
This coulomb stuff is just an arbitrary name given to the measure of electric
charge. We’ll get back to that in a minute. First, note that we are going to run
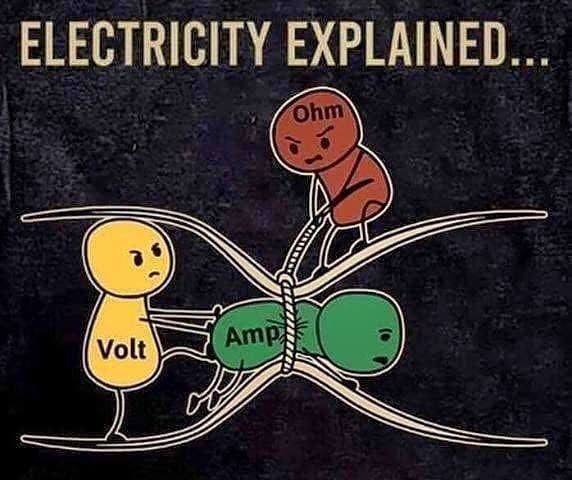
into all kinds of things that have these arbitrary names. Names like Ohms, Volt,
Ampere, Henry, Farads. All these names have quantities of “stuff” associated
with them. The “stuff” is all different, which is why they have different names
and they are not equivalent. Although, in some cases, one type of quantity of
stuff may be expressed as some other quantity of other stuff, both related by
some numeric quantity. We’ll try to point those cases out when we run into them.
For now, note that each of these names refer to some person who, back in
history, discovered and then described the physical characteristics of a
particular property of material, a process, or an effect. Their name has then
been ascribed to each of these properties in order to be able to conveniently
refer to them. Each of these historical people have extremely interesting
stories associated with them. How they first noticed the property of matter that
became associated with their name, how they developed techniques to quantify or
measure the property, and subsequently, how their discovery led to our further
understanding of the nature of the characteristics of the universe. There are
lots of these names and it will become important to understand each of the
properties of matter that the name refers to so that eventually, you have a
complete understanding of the full nature of the sciences associated with
electronics. Although there are lots if these names, they will become familiar
and their meaning will eventually be comfortable to you. I urge you to explore
the stories of each of the people whose names we will be using. Almost every one
of the stories is interesting and the remarkable thing is just how long ago
their discoveries were made. Think of how observant, then curious and clever
they were to have worked out their contributions to knowledge of the universe.
The question is, will you be as clever and make such an important a contribution
as any of them? Note that as time passed, nearly each of those people studied
the discoveries of their predecessors, came to understand the previous
discoveries, then used that previous information to formulate their own
contributions. Nobody understood it all to begin with. Interestingly, just think
about all the information that is available to you, right now, that those folks
did not have available to them in their lifetimes!
Ok, let’s get back to the coulomb. Wait a minute, who says the electric charge
of an electron is -1.602 x 10-19 coulomb? How did they figure that out? Actually
Robert Milliken figured it out in 1909 by performing his famous oil drop
experiment. The reason its famous is because it finally determined what the
electric charge on a single electron was. How? By determining how many electrons
were in a tiny drop of oil which he was able to suspend in mid-air by applying
opposite electrostatic potentials to two plates which allowed the oil drop to
float in mid-air. By carefully measuring the electrostatic potential, and
knowing roughly how many molecules of oil were in the drop and thus how many
protons and electrons, it was possible to calculate the amount of charge that
had to be applied to all of the electrons to get the molecules of oil to
overcome the attraction of the force of gravity. Dividing the amount of charge
by the number of electrons results in the charge contributed by a single
electron.
How many molecules of oil were in the drop? Well, if we make a whole bunch of
drops, count how many of the drops it takes to comprise a given volume, say ten
cubic centimeters, then divide 10 cc by that number of drops, we can pretty
closely determine the volume of a drop. Since they could determine the density
of the oil, the mass of a droplet could be determined. The mass then reveals the
number of molecules. Knowing the number of molecules and the chemical formula of
the oil tells us how many atoms of each element make up the molecules and then
how many protons and electrons are contained in that volume of oil within the
droplet. Very clever progressive reasoning. Won him a Nobel prize.
It gets a bit more complicated and involved to fully explain a coulomb because
it also involves forces in different directions or vectors, so let’s try to
simplify it a bit and leave the full explanation for you to discover some other
time. In about 1777, a French physicist named Charles Coulomb developed a device
called a torsional balance which is a very sensitive mechanism for measuring the
amount of force applied to a fine fiber, spring, or wire. By using the torsional
balance, Coulomb determined that electrically charged materials – probably
copper or brass spheres (with a static charge applied by rubbing those materials
with probably cat fur) repelled each other with forces that varied directly
(which means that the effect is mathematically described within the numerator of
an equation) by both the amount of charge that was on the material (determined
probably by the number of times he rubbed the material with the fur), as well as
inversely (the effect is is described in the denominator of an equation) by how
far apart two pieces of the materials were. In fact, the repellant forces
decreased by 1/4 when the distance doubled and by 1/16 when the distance
quadrupled. So, in 1785 he was able to say that the repellant forces varied
inversely as the square of the distance between them. That’s about all that
could be determined back then. It remained a while (actually 1861 when published
by James Maxwell) before it was noted that the electric charge (the value in the
numerator) influenced things at the speed of light (determined by a number of
investigators moderately accurately between 1676 and 1862) and how well the
materials involved could become magnetic (called magnetic permeability and
described by mathematics developed by Oliver Heaviside in 1885). Together then,
Coulomb’s initial observations were refined by many famous names that appear in
physics until a value for the unit electrical charge on an electron was finally
determined in 1909.
And there we have it. We have learned how physicists figured out the first
property of electricity: current. And what current really is: the quantity of
unit electric charges flowing past a point in a given amount of time. One ampere
or amp of current consists of 6.2422 1018 electron’s worth of unit charges
flowing past a point in one second. The ampere was named for Andre-Marie
Ampere, a French professor of physics and chemistry born a year before America
declared its independence. Ampere demonstrated the relationship between current
flow and magnetics by showing that two parallel wires attracted or repelled each
other proportionally depending upon the length of the wire and the current they
carried. The ampere is abbreviated “A” or called amp and is usually referred to
as “I” in equations. Other values may be expressed as milliamperes, milliamps,
or ma (1 thousandth of an ampere), or microamp (1 millionth of an amp). Next
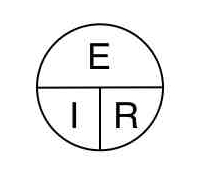
we’ll explore the volt and then we will have learned about two of the three
elements of the most basic equation of electronics, ohms law. From there,
everything else is down hill.
73… W3SEH